Looking Back at the Year in Research: 2019
For years, doctors didn’t have much in their arsenal to help extend the lives of their pancreatic cancer patients.
There were a few chemotherapy agents, but none of them worked well. And it didn’t look like anything in the pipeline was going to make much of a difference. Plus, scientists didn’t know much about the molecular underpinnings of the disease, the basic biology that makes pancreatic cancer tick. Fortunately, over the last several years there have been major improvements in therapies and drugs approved that can potentially make a difference in the lives of some patients. Simultaneously, researchers are learning more about pancreatic cancer biology, the so-called tumor microenvironment, which can lead to improved laboratory models of the disease and new therapeutics aimed at specific targets.
There is no doubt the entire field of pancreatic cancer research is moving forward. And 2019 was no exception. At year’s end, it’s important to look back at what you’ve accomplished, and how much work you may still need to do. Pancreatic cancer research is no exception to that adage. At Let’s Win, we look forward to bringing you news of more scientific achievements in 2020. Our hope, and yours, is that as research moves forward and is then translated into better patient therapies, pancreatic cancer will be as successfully treated as many other forms of the disease we call cancer.
A Place for PARP Inhibitors
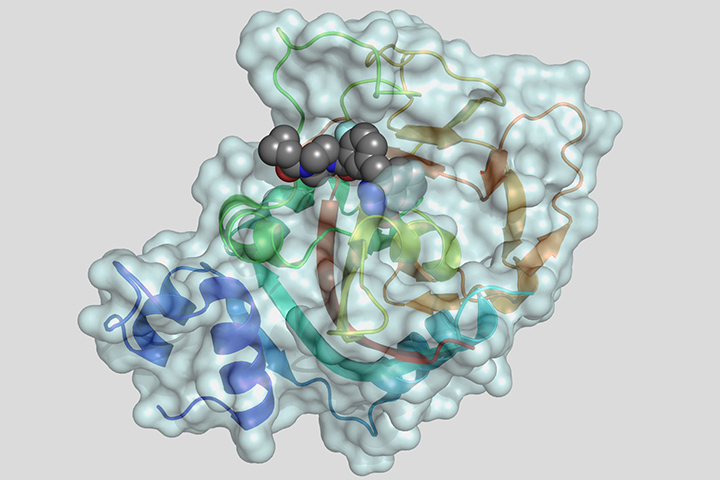
At the 2019 ASCO annual meeting held earlier this year, researchers involved in the randomized phase III POLO trial showed maintenance therapy with the PARP poly(adenosine diphosphate–ribose) polymerase inhibitor olaparib significantly delayed the progression of metastatic pancreatic cancer in patients with germline BRCA gene mutations compared with a placebo. In December 2019, in a 7 to 5 vote, the FDA’s Oncologic Drugs Advisory Committee recommended olaparib as first-line maintenance therapy for patients with germline BRCA-mutated metastatic pancreatic cancer whose disease did not progress after first-line treatment with platinum-based chemotherapy.
PARP is an enzyme that plays a significant role in DNA repair. By blocking (or inhibiting) this enzyme with a PARP-inhibiting drug, DNA inside the cancerous cells is less likely to be repaired, leading to cell death and possibly a slow-down or stoppage of tumor growth. Tumors may also have an increased sensitivity to chemotherapy. This type of treatment is called targeted therapy, because it attacks only the cancer cells and not the normal cells.
According to the research, at 6, 12, 18, and 24 months after randomization, patients who received olaparib were at least twice as likely to have no disease progression compared with those who received a placebo, with olaparib reducing the risk of disease progression by 47 percent. Median progression-free survival on olaparib was 7.4 months, compared to 3.8 months for patients receiving a placebo. After one year, 33.7 percent of patients receiving olaparib showed no signs of disease progression, compared to 14.5 percent of those on a placebo. After two years, 22.1 percent of patients receiving olaparib had no disease progression compared with 9.6 percent of those on a placebo.
GENERATE Study Opens
In 2019, a new study called GENERATE came to fruition, and genetic researchers are understandably excited. GENERATE is a cancer interception study that stands for GENetic Education, Risk Assessment, and TEsting. The ultimate goal of the study is to improve genetic testing and cancer prevention in family members of pancreatic cancer patients with inherited mutations. The multi-site study will also measure how different methods of genetic education increase the rate of genetic testing in these families.
Artificial Intelligence Shows its Potential Power
Lustgarten Foundation-funded researchers at Johns Hopkins (Baltimore, Maryland) have released promising results showing that computers can be trained to detect pancreatic tumors in CT scans, as reported in the American Journal of Roentgenology. Pancreatic cancer can be subtle and can be missed even by experienced radiologists reading CT scans, the most common imaging modality used for the initial evaluation of suspected pancreatic cancer. The research focuses on artificial intelligence, with the goal of training machines (“machine-learning”), to help improve the odds of detecting pancreatic cancer earlier. This field of study, called radiomics, aims to extract many quantitative features from medical images using data-characterization algorithms, with the goal of identifying characteristics of the disease that are missed by the naked eye.
In other artificial intelligence news, a new assay called CompCyst is also showing promise. CompCyst, developed by researchers at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, could potentially change how doctors manage these pancreatic cysts, according to a study published in the journal Science Translational Medicine.
Pancreatic cysts are very common, and the sheer number of people being diagnosed with them is growing, due to more people undergoing abdominal imaging for a variety of problems. Pancreatic cysts are then found incidentally. Although most cysts don’t progress to cancer, some mucin-producing cysts can become malignant. Methods for figuring out what type of cyst a patient has and which of these mucin-producing cysts may be headed toward malignancy are imperfect. This results in extensive monitoring, imaging, and too often, complicated and unnecessary surgeries. According to study results, CompCyst was more successful than other approaches at differentiating patients who might or might not benefit from surgery or require further monitoring. The researchers hope to launch a prospective study of about 1,000 patients in 2020.
Radiation Therapy: New Clinical Guidelines
In 2019, a new clinical guideline from the American Society for Radiation Oncology (ASTRO) provided recommendations on the use of radiation therapy to treat pancreatic cancer patients. With newer therapies and the emergence of stereotactic body radiation therapy (SBRT) as a treatment modality, pancreatic cancer treatment has undergone changes, which led the group to develop its first guideline on this topic. The guideline included recommendations as to when radiation treatments were appropriate. Optimal dosing, timing, and fractionation for these treatments as well as ways to mitigate side effects were also included. The guidelines were published online in Practical Radiation Oncology, the clinical practice journal of ASTRO.






