MRI-Guided Radiation: A New Option for Pancreatic Cancer Treatment
Anyone who has attempted to play the game Operation knows how harrowing it can be to try to extract the game pieces from the small, enclosed spaces without touching its highly sensitive surroundings and setting off the buzzer.
Now imagine doing it with invisible rays of powerful energy instead of tweezers. Blindfolded.
Pancreatic cancer has long presented a challenge to radiation oncologists, who are barely able to see the tightly-protected organ, never mind deliver sufficient doses of radiation without causing collateral damage to the organ’s delicate neighbors.
A new therapy being tested at a handful of locations across the United States may finally make radiation a viable option for people with locally advanced pancreatic cancer. Initial results show promising potential for improving survival rates.
A SMART Start
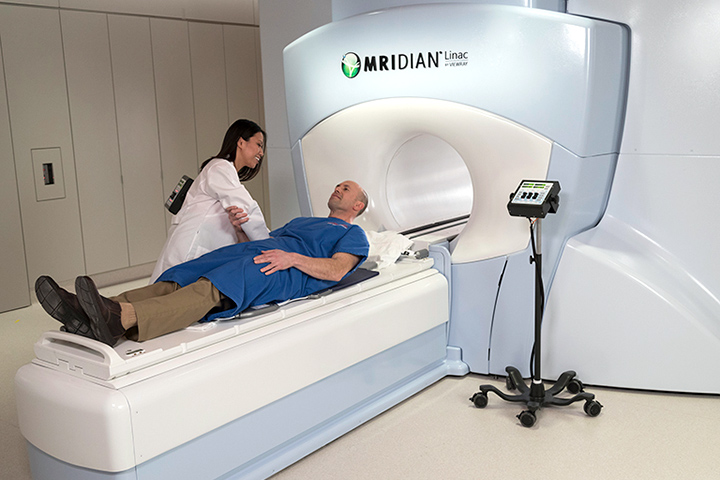
Marrying imaging and precision treatment delivery, new technology approved by the U.S. Food and Drug Administration (FDA) within the last two years allows doctors to see the treatment area with real-time magnetic resonance imaging (MRI). The doctors can then deliver precise radiation at the same time, while more effectively protecting surrounding healthy tissue.
During radiation treatment, it is natural for a tumor to shift, or for the body’s organs to move and change shape, even during normal breathing patterns. Because the MRI images show organs and structures inside the body in real-time, the new system allows physicians and physicists to adapt to changes in the patient’s anatomy and make adjustments to the position and dose of radiation.
“It’s been game changing,” says Parag Parikh, M.D., who was an early adopter of the treatment while at Washington University (St. Louis, Missouri). “The result is a treatment that is tailored just for you. This is personalized radiation treatment at its best.”
Parikh now leads the GI Radiation Oncology program at the Henry Ford Cancer Institute in Detroit, Michigan, and has enrolled the first patients in a new, five-year Stereotactic MRI-guided On-table Adaptive Radiation Therapy (SMART) trial.
Researchers at sites with this technology, such as UCLA (Los Angeles), Washington University, University of Miami, Miami Baptist University of Wisconsin, and Weill Cornell Medicine (New York), will enroll 133 patients with borderline resectable or inoperable locally advanced pancreatic cancer.
“This is the largest open study with radiation therapy in this disease, and the only one with ablative radiation to destroy the tumor,” Parikh said.
Trial participants will receive five treatments at a total dose equivalent to doses given during curative lung and cervical cancer treatment, but nearly twice what is usually attempted in the abdomen.
The therapy can be completed in less than half the time, too, with treatments given either every day or every other day, and not lasting more than two weeks. This is in comparison to conventional radiation treatment lasting five weeks. Parikh hopes this will make it more accessible for those who have to travel to a treatment center with the equipment. The shorter schedule means patients are able to undergo supplementary treatments, such as chemotherapy or surgery, more quickly, which can improve outcomes even further.
First introduced at Washington University in 2014, the technology’s popularity is growing, with 20 to 25 machines now scattered around the world, Parikh said.
At almost double the price of traditional linear accelerators, the MRI-guided machines are expensive, but not prohibitively so, and Parikh said he is confident the technology’s adoption will continue to grow as more clinical evidence of its therapeutic benefits emerges. Parikh, who holds a role with the National Cancer Institute (NCI) pancreatic cancer task force, said the results of the SMART trial may influence future practice.
One barrier that may be difficult for some patients to overcome is claustrophobia. Patients often have to spend an hour or more inside the constricted space of the MRI machine. Parikh said the healthcare team at his center takes extra steps to ensure patient comfort, such as pain and nausea medications. By paying such close attention to the state of patients’ stomachs, these measures often have the added benefit of reducing some of the side effects, he added.
MRI-Guided Radiation in Action
At Weill Cornell Medicine, patients play an active role in their treatment, and this has been a big bonus for John Ng, M.D., Assistant Professor of Radiation Oncology. “What is powerful about this technology is that it’s very visually apparent,” Ng said. “We let the patients see the videos of their pancreas, stomach, and bowel in motion, and how their breathing can affect the motion. Their eyes light up as they realize what is going on inside their abdomen.”
The patients also work with a team of doctors, physicists, dosimetrists, and therapists to coach and monitor their breathing to help get the tumors in the right stable spot as the radiation is delivered.
Both Parikh and Ng said the treatment has been very well tolerated in the patients they have treated so far, in many cases with less toxicity than the lower, prolonged radiation doses. Early results have also been encouraging, with excellent safety profiles, longer median survival rates (nearly double, from 14.8 months to 27.8 months in one retrospective study of 42 patients with inoperable tumors) and a few stellar success stories.
Ng has treated 15 pancreatic cancer patients since starting the therapy in May 2018. One of them was initially unable to get surgery due to the size and location of her cancer. After radiotherapy on the MRI-guided machine, her tumor shrank from 3 cm to just 3 mm.
As a physician-scientist, Ng is also excited about the opportunities the technology may present for cancer research. The images collected can provide biological and functional information about the tumor and how the tumor changes over the course of radiation treatment. It potentially can show where the tumor is metabolically active, for instance, or which tumor types may be immunogenic.
Ng is keen to see how patients with certain tumor mutations and genetic biomarkers may respond to the new treatment, and whether radiation could be used in combination with other drugs like PARP inhibitors or immunotherapy checkpoint inhibitors to make those therapies more effective. He is studying the “abscopal effect,” where localized radiation therapy sometimes elicits out-of-target tumor responses, such as shrinking metastasis tumors at other sites in the body. His colleagues at Weill Cornell Medicine, such as Silvia C. Formenti, M.D. and Sandra Demaria, M.D., have been leaders in demonstrating these effects in breast cancer patients, and Ng is hopeful that pancreatic cancer patients might respond in a similar way, akin to an in-situ vaccine effect.
“We need to do all we can to improve treatment options for pancreatic cancer patients,” Ng said. “Until now, the results from radiation therapy was like getting a single when we needed a home run. We are hopeful that this technology will help provide some home runs.”






