Testing the Addition of a New Drug to Radiation Therapy for Localized Pancreatic Cancer
Could a new drug targeting cellular DNA repair response enhance the effectiveness of targeted radiation therapy?
One of the strategies that doctors use to treat cancer is to cause DNA damage, for example through radiation therapy. The new drug peposertib blocks some of the enzymes involved in DNA repair response. Researchers are testing whether combining this drug with high-dose radiation therapy works better than radiation alone for patients with pancreatic cancer that cannot be removed by surgery and has not spread to other parts of the body (localized).
What Is Peposertib?
Radiation therapy causes double-strand breaks (DSBs), DNA damage with lethal consequences for cells and organisms if left unrepaired. Adding drugs that block DNA repair could help increase the susceptibility of DNA-damaging agents and expedite the death of cancerous cells. DNA-dependent protein kinase (DNA-PK) plays a crucial role in the repair of DNA double-strand breaks. Peposertib (M3814) is a new drug that inhibits DNA-PK.
How the Trial Works
This phase I/II trial studies the side effects and best dose of peposertib to see how well it works when given together with radiation therapy. The study will also look at the difference in progression-free survival and two-year overall survival between patients treated with the radiation and peposertib combo compared to patients treated with radiation alone.
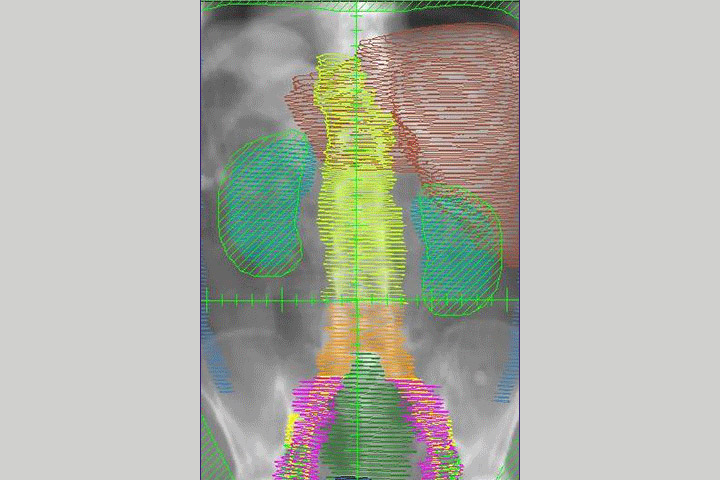
In the phase I dose escalation part of the study, patients undergo hypofractionated radiation therapy—high doses of radiation over a short period of time—as well as escalating doses of peposertib for 14 days.
Once a good dose of the drug is determined, the trial will progress to phase II, where patients will be randomly assigned to two groups. The first group will undergo the radiation and peposertib combo for two weeks; the second will undergo radiation and a placebo pill. Participants will submit blood and tissue samples, as well as CT and MRI scans.
The trial is open to those with pancreatic adenocarcinoma that has not spread to other parts of the body. Patients should have received chemotherapy with FOLFIRINOX or gemcitabine/Abraxane prior to the start of the trial.
We encourage you to consult your physicians for clinical trials that may be right for you. The website ClinicalTrials.gov provides more details about this trial as well as many others. You can visit the Let’s Win Trial Finder for a list of all active pancreatic cancer clinical trials.