A New Clinical Trial Leads to No Evidence of Disease

- A long time to diagnosis
- The first patient on a new protocol
- FOLFIRINOX and radiation before the Whipple
- Gemcitabine after surgery
On May 23, 2013 my life changed forever.
I had been sick for almost six months, with bloating after eating, heartburn, and light grey stools. It was so uncomfortable after eating, that I chose not to eat! Every PET scan, CT scan, MRI, and biopsy seemed to rule out “the really scary things” according to my GI doctor. Fortunately, this doc was persistent and knew my symptoms should not be ignored. Another MRI was ordered, and in this one, they were able to find the tumor. I had pancreatic cancer.
Outlining a New Protocol
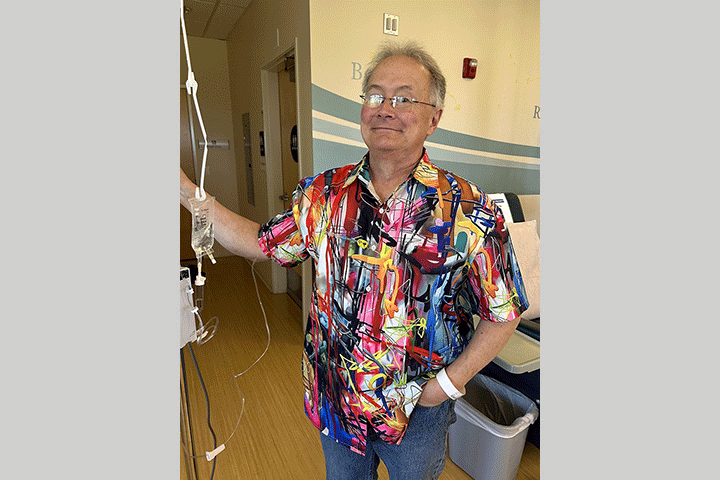
We went to MD Anderson Cancer Center in Houston, Texas, for treatment. My treatment team, led by surgeon Matthew Katz, M.D., designed a new protocol for my case—I was to be patient 1 in a new clinical trial. We would begin with eight weeks of intravenous FOLFIRINOX to be administered in four doses—one every other week. Following the FOLFIRINOX I was to receive daily radiation for six weeks in conjunction with the chemotherapy drug capecitabine. At the conclusion of these treatments, I was to be scanned to determine if I was a candidate for the ultimate surgery, the Whipple. Once I had recovered from the Whipple, I received another four months of chemotherapy with gemcitabine. All of my treatment had to be administered in Houston. Our insurance covered the treatment once our deductible had been met.
Based on the scans, Dr. Katz felt my cancer had progressed to around stage IIb. The tumor was in the head of the pancreas and wrapped around the portal vein. Wasting no time, a peripherally inserted central catheter (PICC) line was inserted and we began treatment. FOLFIRINOX is a “cocktail” of leucovorin calcium, also known as folinic acid (FOL), fluorouracil (F), irinotecan (IRIN), and oxaliplatin (OX). The chemo cocktail was administered in clinic where I was closely monitored. Then I was released with a pump to continue the chemo for another 72 hours. I took the next week “off” to recover, and returned for another dose the week after that.
Treatment with FOLFIRINOX was the most difficult part of the protocol for me. My team did a tremendous job of controlling many of the side effects: nausea, diarrhea, vomiting, weakness. Peripheral nerve damage can occur with the oxaliplatin, so I would wear driving gloves everywhere to counter the sensitivity.
The second stage of my protocol was radiation plus capecitabine pills. I was told to expect to feel like I had the worst case of flu ever. I was pleasantly surprised. While I rested and slept much more than normal, it was much easier than the FOLFIRINOX in terms of side effects. Throughout the entire trial, I never lost my hair, although, it did thin some during this second phase.
Time for the Whipple
On November 8, 2013, I had the Whipple surgery. They cut out about half of my digestive tract and 28 lymph nodes. They removed the portion of the portal vein where the tumor had contact and “stretched” the two loose ends to reconnect it. It was an extremely long surgery—it took the first three hours just to get to the pancreas!
After a night in an Intermediate Care Unit, I was sent to MD Anderson’s Whipple ward for a week. I was given clear liquids on day 2, full liquids on day 3, and my first “meal” on day 4: one teaspoon of Cream of Wheat. This is done to get what remains of your digestive tract functioning as quickly as possible. Additionally, I was up and walking within the first 24 hours. I had heard from several “Whipple Warriors” that walking is crucial to rapid recovery after a Whipple—this was true for me as well. On Day 3, Dr. Katz and his team came in with the pathology report: everything was stage 0! The chemotherapies and radiation had killed all of the tumor! On day 10, I was allowed to go home to Austin. I remained on a fat-free, sugar-free, fiber-free diet for the next eight weeks.
I returned to Houston after Christmas for scans and implementation of the last phase of the clinical trial protocol. I received intravenous gemcitabine each week for three weeks, followed by a one-week break, for four months. This treatment was administered in Houston. Gemcitabine was the easiest part of the protocol for me.
My Life, Post-Treatment
It will be four years since my diagnosis and three years since completion of the clinical trial protocol in May 2017. I take Creon, a prescription drug that provides digestive enzymes, with meals and snacks so I can digest proteins and fats. There is a trick to taking the Creon for it to be effective—you must take it simultaneously WITH the first bite of food. I must take a multitude of supplements and vitamins to counteract malabsorption of nutrients. I am not diabetic. I exercise almost daily.
For the past three years, I returned quarterly for follow-up scans. They all remain clear, with “No Evidence of Disease.” I am now on a six-month follow-up schedule and will be for a very long time.
In light of this journey, I have come to believe that every day is a gift! My doctors say that I am a miracle, but I know miracles happen every day at MD Anderson.
Read about the latest advances to the Whipple procedure in the story “An Update on the Whipple Procedure,” and learn how to eat properly after surgery from the Disease Management article “How to Eat after A Whipple Procedure.”