Making Sure Patients Get Genetic Testing

When someone hears the words “you have pancreatic cancer,” the next steps come fast.
Treatment decisions often need to be made within days, not weeks. And according to the National Comprehensive Cancer Network (NCCN), genetic testing is standard of care for every newly-diagnosed patient, not just those with a family history. That means that one of the first steps after diagnosis should be genetic testing. This shift is reshaping the way doctors understand and treat pancreatic cancer, and it has important implications not only for patients but for their entire families.
However, not all doctors know to offer the testing, and not all patients understand why testing is so key. But understanding of the importance of this test is spreading.
A Team Approach that Starts on Day One
At UPMC Hillman Cancer Center in Pittsburgh (Pennsylvania) and other comprehensive cancer centers, genetic testing is built directly into the intake visit for newly-diagnosed patients.
These centers use a multidisciplinary model, which typically includes:
- Genetic counselors
- Medical oncologists
- Surgical oncologists
- Radiation oncologists
- Dietitians
- Clinical trial coordinators
- Practice nurses
- Insurance/financial coordinators
Genetic counselors are an important part of this team. They can help explain why testing is needed for treatment. “It’s important that patients understand what may or may not be found, and the implications,” says medical oncologist Janie Yue Zhang, M.D., who cares for pancreatic cancer patients. “Genetics are very complicated.”
For example, a patient may carry only one nonfunctioning copy of a gene and have completely normal health—until the second copy of the gene is lost due to chance, age, environmental exposure, or another unknown factor. Even when a mutation is inherited with a 50 percent chance, the actual personal risk remains difficult to define.
Genetic counselors help patients make sense of these nuances. And insurance specialists can help navigate coverage, which—although genetic testing is recommended by national guidelines—can still vary. In some cases, a self-pay option for a gene panel is actually cheaper than going through insurance.
UPMC is not alone. Dozens of major centers across the United States, Canada, and Europe have joined the PRECEDE Consortium, which has built the world’s largest cohort of high-risk people. The group’s mission is bold: increase the five-year survival rate of pancreatic cancer from 10 percent to 50 percent within the next decade by improving early detection and prevention.
Other centers are contributing through the CAPS5 Study, which tracks long-term outcomes in people with inherited risk. Together, these efforts are pushing the field toward a new standard of care: universal germline testing for all newly-diagnosed pancreatic cancer patients, regardless of age or family history.
When Family History Doesn’t Tell the Whole Story
For years, clinicians tested only patients who had a known family history of pancreatic cancer or other high-risk features. Until 2018, the guidance from the NCCN was to test only those of Ashkenazi ancestry or with a family history of Hereditary Breast and Ovarian Cancer Syndrome (HBOC) cancers, which are most commonly caused by mutations in the BRCA1 and 2 genes. Those guidelines were updated seven years ago, but some clinicians are still catching up.
“Family history is not always reliable for identifying who carries a mutation that could predispose to developing pancreatic cancer,” says Zhang. “About 10% of pancreatic cancer has a hereditary or familial component.”
In fact, the most common result of testing is actually no mutation at all. The majority of pancreatic cancer cases cannot yet be explained by known genetic factors. Today’s testing panels examine around 40 to 90 genes, not the whole genome, and they are continually expanding. Scientists are learning new things all the time—the significance of BRCA gene mutations were not truly understood until about 20 years ago, for instance.
Many patients receive results that include a variant of unknown significance (VUS)—something to be logged, watched, and studied over time. Large academic centers with strong research programs—such as those participating in the PRECEDE Consortium or the CAPS5 early detection study—store blood samples and tissue so researchers can revisit these unanswered questions. This kind of long-term, large-scale data sharing led to discoveries like the role of ATM mutations in pancreatic cancer.
Genetics Can Affect Treatment—Immediately
One of the biggest reasons to test early is that the results may help guide treatment. BRCA1/2 and other mutations in DNA-repair genes can make tumors especially sensitive to platinum-based chemotherapy, which is often used in first-line treatment. “We want to resolve this ASAP to make treatment decisions,” explains Zhang, also an assistant professor at the University of Pittsburgh School of Medicine. And time is of the essence.
“Unlike many cancers, in pancreas cancer, you get a very short window to act and make decisions,” says Randall Brand, M.D., a physician–scientist at UPMC and professor of medicine at the University of Pittsburgh involved in early detection research. “The information that early genetic testing provides could help guide treatment selection. The patients like it, and we have found it to be very effective.”
A Ripple Effect for the Whole Family
Genetic testing is not just about the patient sitting in the exam room. “There can be a ripple effect of a pancreatic cancer diagnosis on the entire family,” adds Brand.
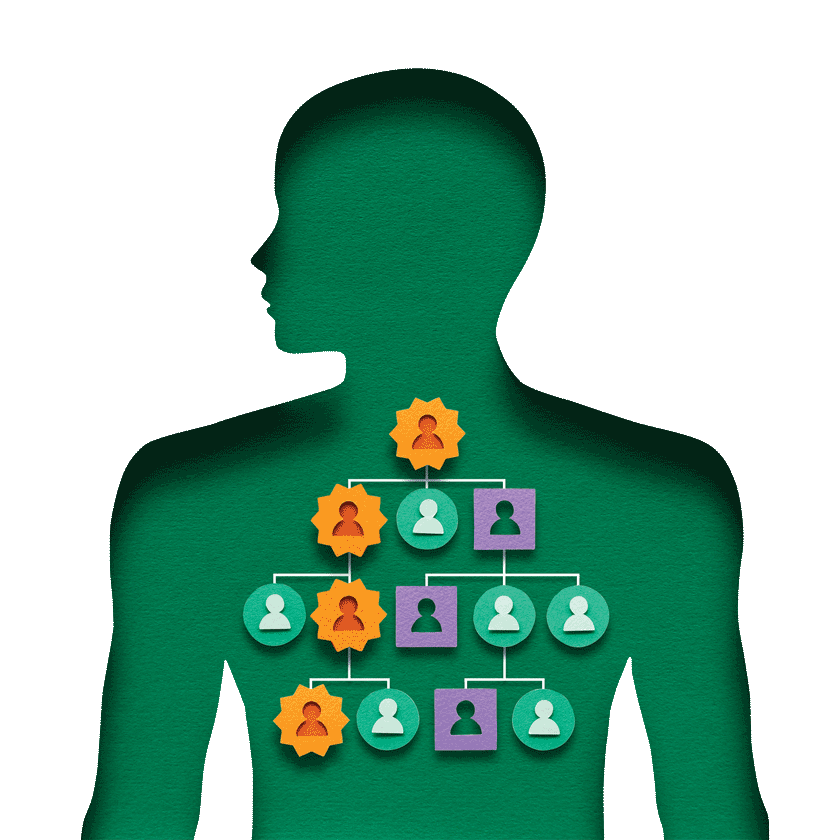
If a mutation is found, relatives may also carry an increased risk for pancreatic cancer—or for other cancers, such as breast, ovarian, or prostate cancer, depending on the gene. At UPMC, risk assessments for family members are done free of charge, though the medical genetics clinic cannot reach out directly; the patient must initiate the referral.
These programs are seeing strong participation. Family members often feel that knowledge is power—a way to catch cancer early or prevent it altogether. A BRCA mutation in a pancreatic patient, for example, may lead a sister to get a breast MRI or a father to get prostate screening, years earlier than they otherwise would.
And for many patients, the decision to get tested is motivated as much by their family as by their own care. “They understand that one test in them could spare a lot of family members from going through the cancer journey,” Brand notes.
His patient, Andy Lyons, benefited from years of proactive monitoring due to a family history of pancreatic cancer that took his grandmother and at least two of her sisters, his mother, her twin sister and brother, and his own brother.
“After I lost my brother, I made it my life’s mission to not only avoid the same fate, but to help find a way of stopping the pancreatic cancer killer in its tracks,” Lyons told Let’s Win in this video documenting his journey.
What this Means for Patients and Families
For people facing a new diagnosis, the idea of genetic testing can feel overwhelming. But doctors emphasize that the goal is empowerment—not anxiety.
Early testing may:
- Open access to targeted treatments
- Identify eligibility for precision medicine clinical trials
- Clarify risks for children, siblings, or parents
- Connect families to screening programs
- Advance research for future patients
And importantly, patients do not have to navigate any of this alone.
Most major cancer centers now offer coordinated, team-based support from the very beginning—because decisions made in the first days after diagnosis may shape the entire course of treatment.