CAR T Cell Trial Targets Mesothelin
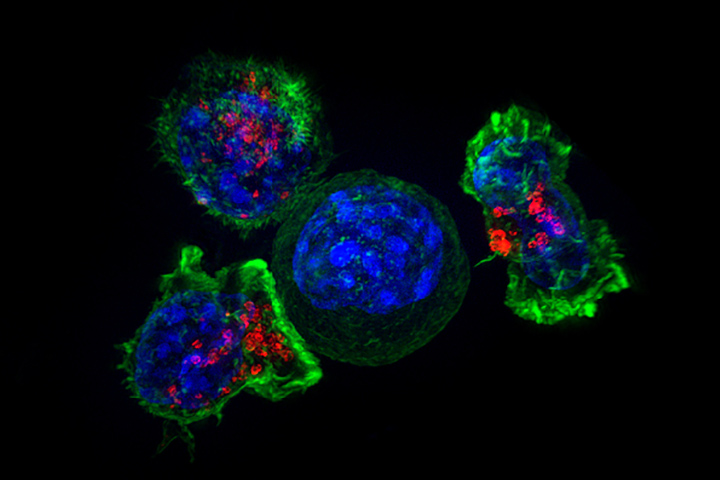
Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths; National Institutes of Health
Immunotherapy holds the mantle of being the next big—or actually the current big—thing in cancer treatment.
But the emerging form of immune treatment called CAR T cell therapy is gaining traction as its potential superstar. Although it has shown some remarkable results in blood tumors like leukemia, solid tumors like pancreatic cancer have been more difficult to treat. Part of the problem is that tumors like pancreatic tumors are immunosuppressive. This means that the body’s T cells face a very hostile microenvironment and have a tough job of recognizing and killing invaders like cancer cells. CAR T cell approaches have also been limited in solid tumors because researchers have had some difficulties in identifying appropriate tumor-specific or tumor-associated target antigens.
Researchers are looking at novel ways to boost the effectiveness of immunotherapy in pancreas and other solid tumors, often by combining agents and by homing in on potential targets. In a new trial at the University of Pennsylvania, researchers are using a specially-designed CAR T cell treatment that targets and kills mesothelin-expressing tumor cells. Mesothelin is a so-called tumor-associated antigen that is expressed in normal cells but is overexpressed in pancreatic cancer and other malignancies.
“There are a lot of reasons to hope that CAR T cell treatment works in (pancreas) cancer, with the main reason being that our patients need better, more effective treatments,” says lead investigator Dr. Mark O’Hara, Assistant Professor of Medicine at the Abramson Cancer Center at the University of Pennsylvania, Philadelphia. “What we need to do is to get over the barriers in solid tumors. We know it can work. We just need to find out how.”
What Are CAR T Cells?
CAR stands for Chimeric Antigen Receptor, and CAR T cell therapy uses a patient’s own T cells that are engineered to attack cancer. T cells are the immune system cells that recognize and then destroy invaders, providing a line of defense against infections as well as cancers. But sometimes this elegant system is out of sync, and T cells don’t always recognize malignant cells or they don’t mount an offensive against them. By rigging a patient’s own T cells into CAR T cells that problem is hopefully overcome.
That’s the simple explanation. But the entire process is much more involved. “From a patient perspective there are some factors that have to be considered, including whether they are appropriate candidates, can withstand treatment, and whether they can stay close to the hospital for several weeks after treatment,” says O’Hara, a medical oncologist whose research focuses on pancreatic cancer, colorectal cancer, GI cancers, phase I trials, and CAR T cell therapies. “The lab process can also be extensive since the T cells have to be genetically engineered to express chimeric antigen receptors, since it’s the CARs that allow the T cells to recognize an antigen on targeted tumor cells. That process can take several weeks.”
Targeting Mesothelin
For this study, the researchers will be using a CAR specific for mesothelin. “In the lab, we’ve seen CAR T cells that have been engineered to target mesothelin be able to destroy mesothelin-positive cells while not touching the mesothelin-negative cells,” says O’Hara. Two small studies examined the safety, feasibility, and activity of a different mesothelin-specific CAR T cell therapy for patients with chemo-refractory pancreas cancer and showed hints of biological activity with no significant adverse events such as cytokine release syndrome. “We are in the very early stages, and researchers involved in pancreas cancer and immunotherapy know that we have a lot of work to do, but we have to raise the bar and find ways of getting CAR T cells to work in the microenvironment of pancreas cancer.”
Compared with the low-level expression in normal tissues, mesothelin is overexpressed in about 30 percent of all cancers, including the majority of pancreas and serous ovarian cancers, among others, O’Hara says. “Mesothelin-directed immunotherapies like CAR T are very promising because there is research showing that we can find mesothelin in almost all pancreatic cancer tissues and cell lines, but there’s virtually none in a healthy pancreas. So that provides a target for immunotherapy.”
About the Study
The phase I study will be evaluating the feasibility of producing, as well as the safety of administering, lentiviral transduced huCART-meso cells in up to three (3) cohorts of participants in a three-plus-three (3+3) design. These cohorts will be used to establish the safety of this investigational product (huCART-meso cells) as well as the target dose level in the target study population.
If safety is established, the study will be further amended to explore the safety of local delivery methods. “This is a small study and we want to enroll about 18 people, and then if we get good results we’ll move on from there with larger studies,” O’Hara says, adding that he gets numerous emails and calls from pancreatic cancer patients both in and outside of the U.S. about this trial. “Patients are interested in immunotherapy and are excited about it. And to be honest, so am I. I think there is some real promise here.”