The Impact of BRCA Is Broader Than Breast and Ovarian Cancer

- Family history of breast and ovarian cancer leads to BRCA2 mutation test
- Searching for a clinical trial after pancreatic cancer diagnosis
- Finding clinical trials for BRCA carriers focused my search
- Doing the homework to find the next trial
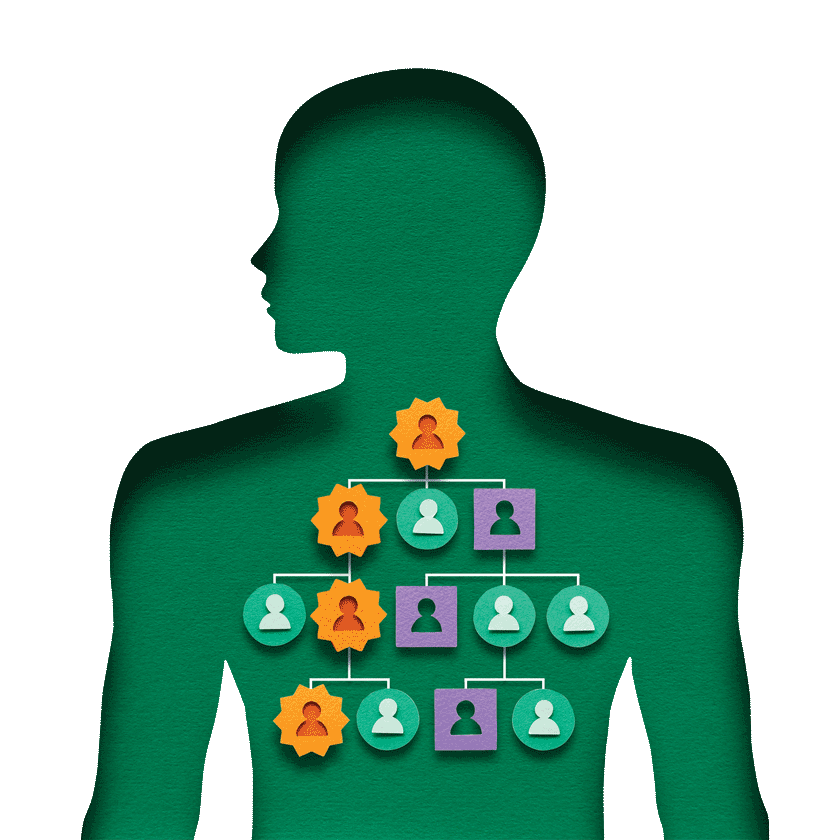
Hereditary breast and ovarian cancer is part of my family history.
My grandmother died from breast/ovarian cancer in 1934; my mother was ten years old. My mother died from the same cancer in 1967; I was nineteen years old.
BRCA2 Found Through Genetic Testing
In 1997, three years after the BRCA genes were identified, my sisters and I participated in a clinical study. The study was designed to genetically test high-risk Ashkenazi Jewish people to determine if they were carriers of the mutated BRCA gene. I tested positive for the BRCA2 mutated gene; my three sisters all tested negative.
Through considerable research, I discovered that the prophylactic removal of ovaries and fallopian tubes would reduce my breast cancer risk by 50%. I had a total hysterectomy in 1998 and entered a surveillance program for high-risk people such as BRCA carriers at the University of Pennsylvania.
FORCE (Facing Our Risk of Cancer Empowered), a national nonprofit organization devoted to hereditary breast, ovarian and related cancers, provided me with education and support. I learned that children of a BRCA positive parent have a 50% risk of carrying the mutated BRCA gene. My two daughters were tested; they were both positive for the mutated BRCA2 gene.
I was diagnosed with very early breast cancer in September 2010 and had a bilateral mastectomy, highly recommended to reduce risk for BRCA positive patients.
Pancreatic Cancer Diagnosis
In October 2015 I started feeling an odd, intermittent pain under my right rib cage. I called my primary physician, who ordered an ultrasound of the abdomen and pelvis. Due to my previous diagnosis of Crohn’s disease and colitis, exactly one year earlier a CT scan had been performed of the abdomen and pelvis. At that time there was no evidence of lesions on the pancreas or liver.
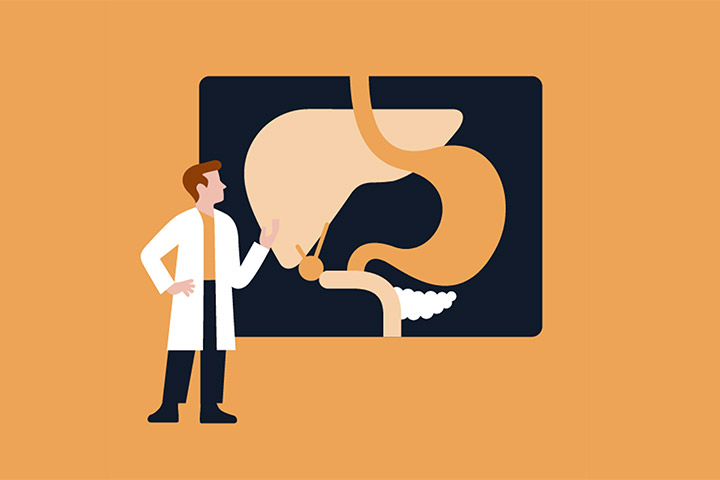
Now, a year later, an ultrasound at Hackensack University Medical Center detected innumerable masses in the liver, and a new CT scan showed a very large tumor in the tail and extending into the body of the pancreas. A biopsy was needed to confirm a diagnosis of pancreatic cancer. Research shows that 5% of BRCA2 carriers develop pancreatic cancer. My diagnosis was pancreatic adenocarcinoma, BRCA2 associated, stage IV, metastasis to liver. Inoperable and incurable. The oncologist recommended a clinical trial to target the BRCA mutation, investigating a promising new class of drug, PARP inhibitors.
Finding the Right Clinical Trial for Me
I started researching and networking with FORCE to explore clinical trials. I was referred to Dr. Michael Hall at Fox Chase, Philadelphia. His clinical trial was POLO: A randomized trial of Olaparib in BRCA mutated Pancreatic Cancer. However, randomization means that I would have a 50% chance of not receiving the investigational PARP drug. I declined the trial.
Dr. Eileen O’Reilly is a prominent pancreatic cancer specialist at Memorial Sloan Kettering Cancer Center in New York. I investigated her clinical trial: The Study of Gemcitabine, Cisplatin +/- Veliparib in patients with Pancreas Adenocarcinoma and BRCA mutation. It was another randomized trial and I again declined.
My next appointment was at Georgetown Hospital with Dr. Michael Pishvaian, principal investigator of The Study of Veliparib in Combination with 5-Fluorouracil and Oxaliplatin in Patients with Metastatic Pancreatic Cancer. Finally! I found a non-randomized trial!!
After another CT scan and biopsy at Georgetown Hospital, I started the clinical trial in November 2015. Treatment included: seven days of veliparib capsules, two in AM and two in PM; oxaliplatin, leucovorin, ondansetron, and dexamethasone (anti-nausea medications), and a 46-hour pump, which infuses fluorouracil.
After about two months, the veliparib was reduced in strength due to neutropenia, low white blood count. The oxaliplatin was reduced after almost five months because of peripheral neuropathy and it was subsequently eliminated due to an allergic reaction. According to Dr. Pishvaian, this occurs frequently among the patients in the study and the majority of patients continue to do well without the oxaliplatin.
After starting treatment, I experienced most of the common major side effects of chemotherapy, including fatigue, stomach and appetite problems, dizziness, low white blood count, and peripheral neuropathy. My side effects have minimized and are now mild.
CT scans are performed every eight weeks during the study and continue to show tumor reduction.
Looking Ahead at More Clinical Trials
Dr. Pishvaian has explained that there will be a time in the future when the disease will start to progress again. At that time, the current clinical trial will end for me and I will need to make other decisions regarding my treatment. I am investigating the possibilities of immunological therapies with Dr. Robert Vonderheide at UPenn and innovative, science-based precision medicine with Dr. Allyson Ocean at NY-Presbyterian Hospital/Weill Cornell.
Over the last several months, in my search for cutting-edge research, I have attended the AACR Special Conference for Pancreatic Cancer, and the Annual BRCA Scientific Symposium at UPenn. Additionally, I was invited to participate in Vice President Joe Biden’s Cancer Moonshot Summit, sponsored by the White House. The collaborative goal developed among the members of the cancer community is to double the rate of progress toward a cure for cancer in the next five years!!
LET’S WIN!!!
Fifteen months after Rona’s story was published, she passed away. During her two-year fight with pancreatic cancer, she used her knowledge gained as a BRCA advocate to actively seek the clinical trials that might give her the best possible outcome. We offer our deepest sympathy to her family.